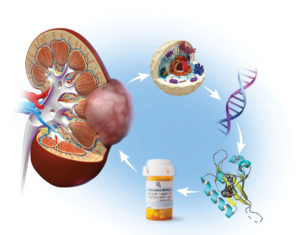
 The FDA approval of belzutifan on Aug. 13, 2021, marks a paradigm shift in the treatment of kidney cancer toward a highly-targeted drug with minimal side effects. It also marks the introduction of a new class of drugs known as HIF-2 inhibitors.
The FDA approval of belzutifan on Aug. 13, 2021, marks a paradigm shift in the treatment of kidney cancer toward a highly-targeted drug with minimal side effects. It also marks the introduction of a new class of drugs known as HIF-2 inhibitors.
The drug evolved from chemical leads discovered by researchers at UT Southwestern Medical Center in the mid-1990s and developed by Peloton Therapeutics in the UTSW Biocenter. Supported by funding from a National Cancer Institute SPORE Award, the UTSW Kidney Cancer Program showed in a 2016 Nature study that the drug PT2977, eventually approved as belzutifan, was able to inhibit HIF-2a in human kidney tumors. PRESS RELEASE